Thursday, 14 February 2013
INTRODUCTION TO SEMICONDUCTORS
Do you like this story?
WHAT IS A SEMICONDUCTOR?
The magic word semiconductor is composed of two words-Semi and Conductor. Semi means not completely while conductor mean something, which can conduct electricity. Everybody is familiar with "Electricity". It is present everywhere; it runs many appliances in your home and outside the home like TV, Bulb, Freeze, and Microwave Oven etc. In simple terms, the current must past through wires so that the electricity can reach all these appliances. So a conductor is nothing but a material having ability to conduct this electricity. Semiconductors conduct electricity to some extent, less than the conductors, how much do you think? Well, it depends on the type of material or it's mixture and size. A semiconductor is a material that has intermediate conductivity between a conductor and an insulator. It means that it has unique physical properties somewhere in between a conductor like aluminum and an insulator like glass. In a process called doping, small amounts of impurities are added to pure semiconductors causing large changes in the conductivity of the material. Examples include silicon, the basic material used in the integrated circuit, and germanium, the semiconductor used for the first transistors.
IMPORTANCE OF SEMICONDUCTOR
To understand the importance of semiconductors let's first understand the difference between electricity and electronics. Both are concerned with generating, transferring, and utilizing electrical energy. The chief difference is that electricity is concerned with using that electrical energy in power applications for heat, light, and motors while electronics is concerned with power control and communications applications such as electronic thermostats, electric motor speed control and radio. Engineering importance of semiconductors results from the fact that
they can be conductors as well as insulators. Semiconductors are especially important because varying conditions like temperature and impurity content can easily alter their conductivity. The combination of different semiconductor types together generates devices with special electrical properties, which allow control of electrical signals. Semiconductors are employed in the manufacture of electronic devices and integrated circuits. Imagine life without electronic devices. There would be no radios, no TV's, no computers, no video games, and poor medical diagnostic equipment.
TYPES OF SEMICONDUCTORS
Semiconductors are mainly classified into two categories: Intrinsic and Extrinsic. An intrinsic semiconductor material is chemically very pure and possesses poor conductivity. It has equal numbers of negative carriers (electrons) and positive carriers (holes). Where as an extrinsic semiconductor is an improved intrinsic semiconductor with a small amount of impurities added by a process, known as doping, which alters the electrical properties of the semiconductor and improves its conductivity. Introducing impurities into the semiconductor materials (doping process) can control their conductivity. Doping process produces two groups of semiconductors: the negative charge conductor (n-type) and the positive charge conductor (p-type). Semiconductors are available as either elements or compounds. Silicon and Germanium are the most common elemental semiconductors. Compound Semiconductors include InSb, InAs, GaP, GaSb, GaAs, SiC, GaN. Si and Ge both have a crystalline structure called the diamond lattice. That is, each atom has its four nearest neighbors at the corners of a regular tetrahedron with the atom itself being at the center. In addition to the pure element semiconductors, many alloys and compounds are semiconductors. The advantage of compound semiconductor is that they provide the device engineer with a wide range of energy gaps and mobilities, so that materials are available with properties that meet specific requirements. Some of these semiconductors are therefore called wide band gap semiconductors.
HOW DOES SEMICONDUCTOR WORKS
Most of the semiconductor devices and chips are created with silicon. The commonly heard expressions like "Silicon Valley" and the "Silicon Economy" come from this fact. In the periodic table, you will find that silicon sits next to aluminum, below carbon and above germanium. Carbon, silicon and germanium have a unique property in their electron structure -- each has four electrons in its outer orbital. This allows them to form nice crystals. The four electrons form perfect covalent bonds with four neighboring atoms, creating a lattice. In carbon, we know the crystalline form as diamond. In silicon, the crystalline form is a silvery, metallic-looking substance. Metals tend to be good conductors of electricity because they usually have "free electrons" that can move easily between atoms, and electricity involves the flow of electrons. While silicon crystals look metallic, they are not, in fact, metals. All of the outer electrons in a silicon crystal are involved in perfect covalent bonds, so they can't move around. A pure silicon crystal is nearly an insulator -- very little electricity will flow through it. You can change the behavior of silicon and turn it into a conductor by doping it. In doping, you mix a small amount of an impurity into the silicon crystal. A minute amount of either N-type or P-type doping turns a silicon crystal from a good insulator into a viable (but not great) conductor -- hence the name "semiconductor."
However electrons are not the only players in the "conduction game"! Another particle plays a major role in conduction in semiconductors. What is this particle? That is also what happens when an electron in a semiconductor jumps from the valence band to the conduction band. It is called a hole. Some electrons and holes play an important role in electrical conduction in semiconductors:
· these electrons are the ones that jumped in the conduction band
· these holes are the ones that are created in the valence band
Electrons have a negative charge. Holes have a positive charge. Electrons and holes are not static: they can move. Holes move more slowly than electrons. When electrons move in one direction, holes move in the opposite direction. This is like cars parked along a street. If one car moves to an empty slot, the empty slot moves the other way.
The cars move to the right, the empty slot to the left. A solitary electron in the presence of a solitary hole will recombine. Only electrons and holes which are free, and hence have not recombined, play a role in electrical conduction.
BY WHICH MATERIALS ARE THEY MADE
All the elements used to make semiconductors appear in Column IV of the Periodic Table or are a combination of elements in columns at equal distance of Column IV on each side.
For example, two elemental semiconductor materials are silicon and germanium from Column IV. Another common compound is gallium arsenide (GaAs), Ga from Column III and As from Column V. The elements for zinc oxide (ZnO) are each two columns away from Column IV, Zn in Column II and O in Column VI. All of these chemical bonds yield an average of four valence electrons per atom. These valence electrons are shared between all the atoms in the silicon crystal. Semiconductors are important because of their electrical properties. Some semiconductors are probably the purest materials on earth. Any trace of unintended impurity atoms can have a drastic effect on those properties. When being manufactured, purity must be very carefully controlled. Intentionally added impurities are called dopants. Dopants are added in a controlled environment and it is known beforehand how many impurity atoms will be added and what the effect will be.
PROPERTIES OF SEMICONDUCTORS
Semiconductors have many useful properties that insulators and conductors do not possess. These properties are based on the fact that an electron can jump from the valence band to the conduction band and vice versa. Temperature can give this little extra energy to an electron and make it jump to the conduction band thus creating a hole in the valence band.
Light can also give this energy boost and create what we call an electron-hole pair: a free electron and a free hole: this phenomenon is called absorption. Photoconductivity is the increase of current in a semiconductor due to the absorption of photons. Light has a dual nature: it behaves as a wave and as a particle. The particle associated with light is called a photon. Photons can have different energies.
· the photons with the right energy are absorbed by the material
· the electrons from the valence band have enough energy to jump to the
conduction band
· the conductivity increases due to the higher number of electrons in theconduction band.
Electroluminescence is the conversion of electrical energy into light. Let's consider electrons in the conduction band. These electrons are in an excited state: they have gained some energy to jump to the conduction band.
· they release the extra energy that they have
· this energy is emitted as a photon
Photons emitted by electroluminescence come out in random directions: this type of light is called incoherent light. For instance light from a light bulb is incoherent. Stimulated emission is a little bit like electroluminescence except that it is not a spontaneous process: the excited electron is forced into jumping back to the valence band and emitting a photon.

This post was written by: Author Name
Author description goes here. Author description goes here. Follow him on Twitter

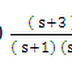







0 Responses to “INTRODUCTION TO SEMICONDUCTORS”
Post a Comment